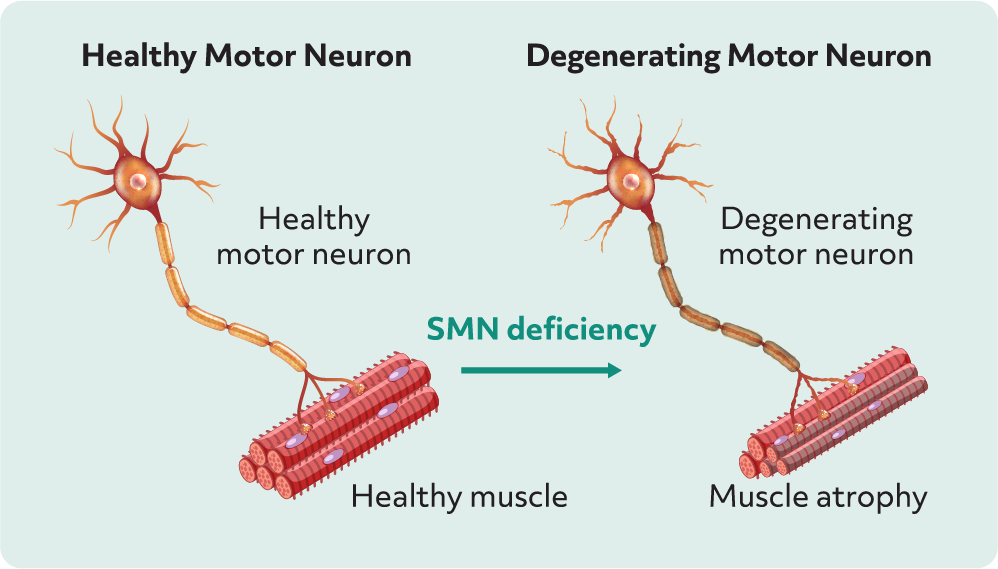
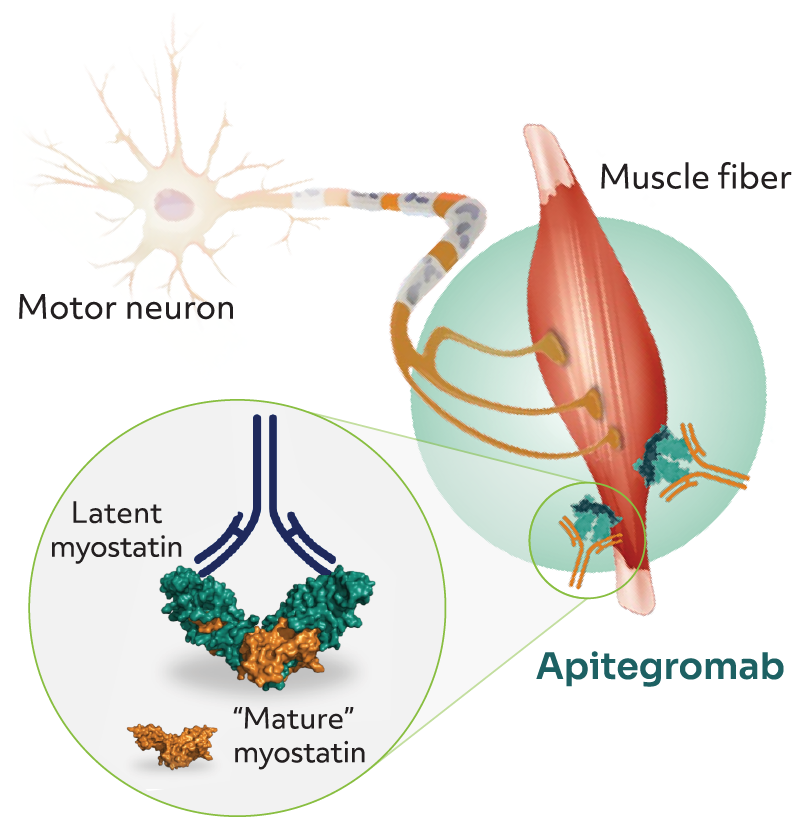
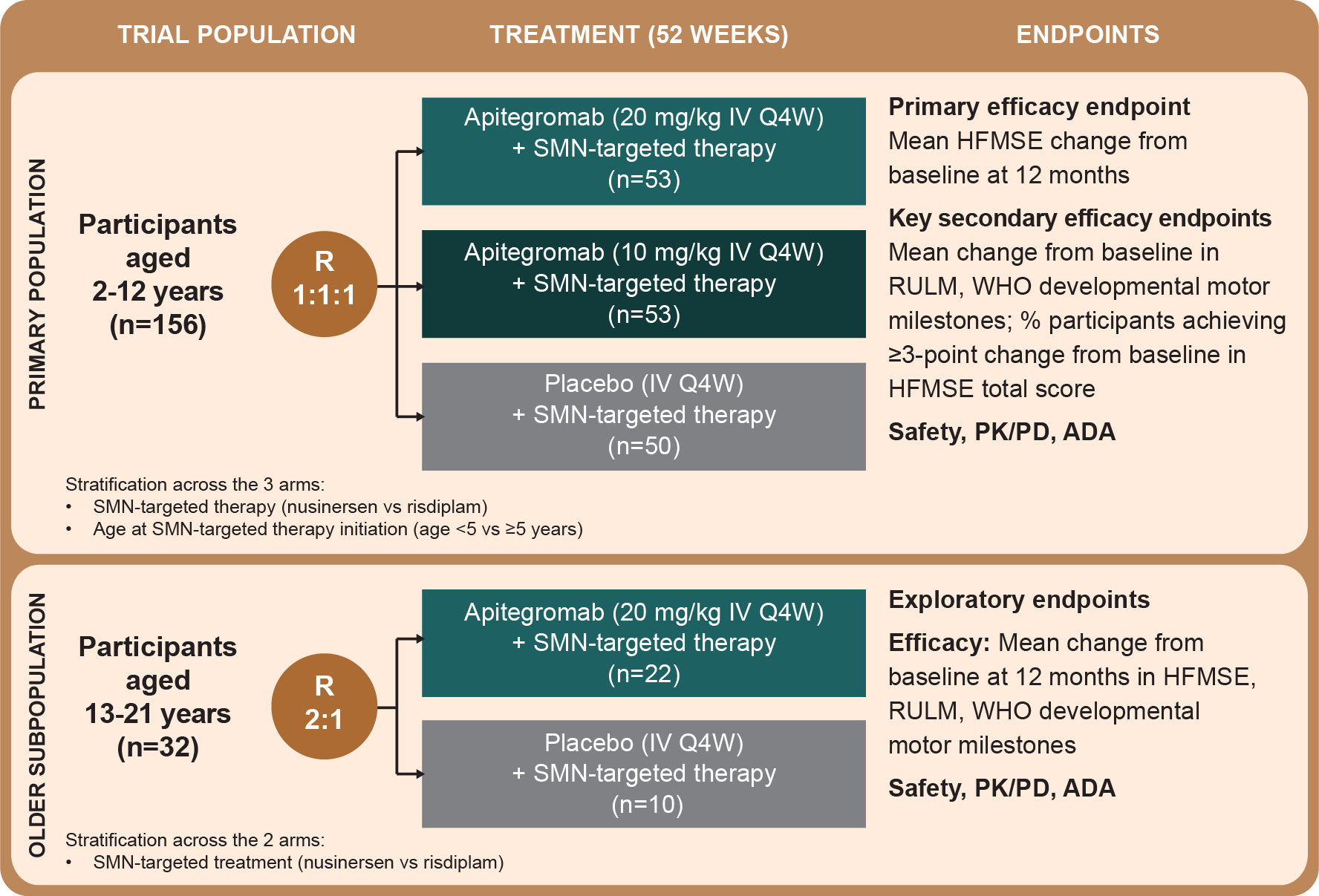
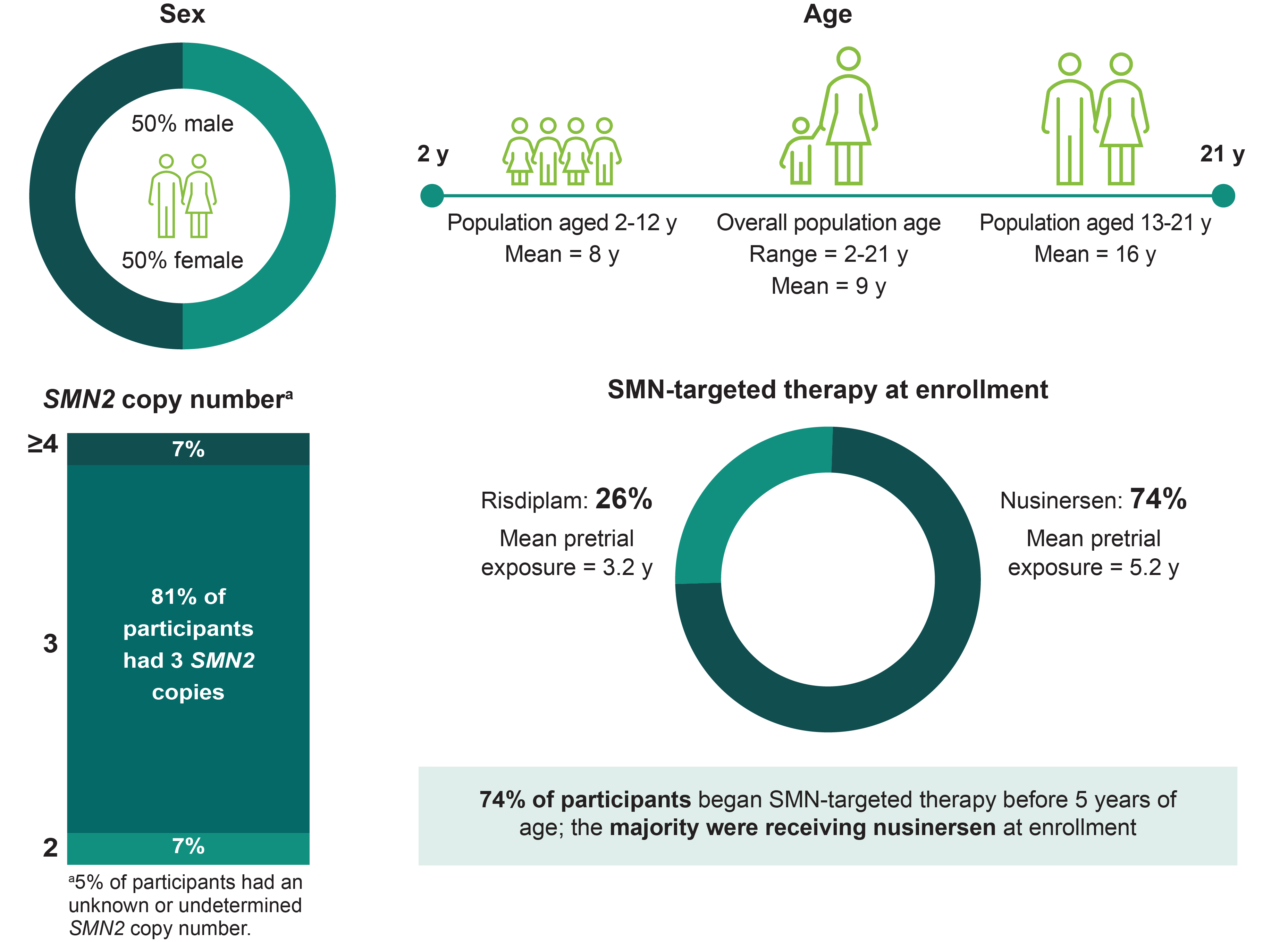
A limitation of this study was that certain patient types were excluded, including those:
- With severe contractures or scoliosis, as HFMSE outcome assessments can be confounded
- Aged <2 years
- With severe or mild weakness, to avoid known ceiling or floor effects on outcome assessments
- Previously treated with onasemnogene abeparvovec-xioi
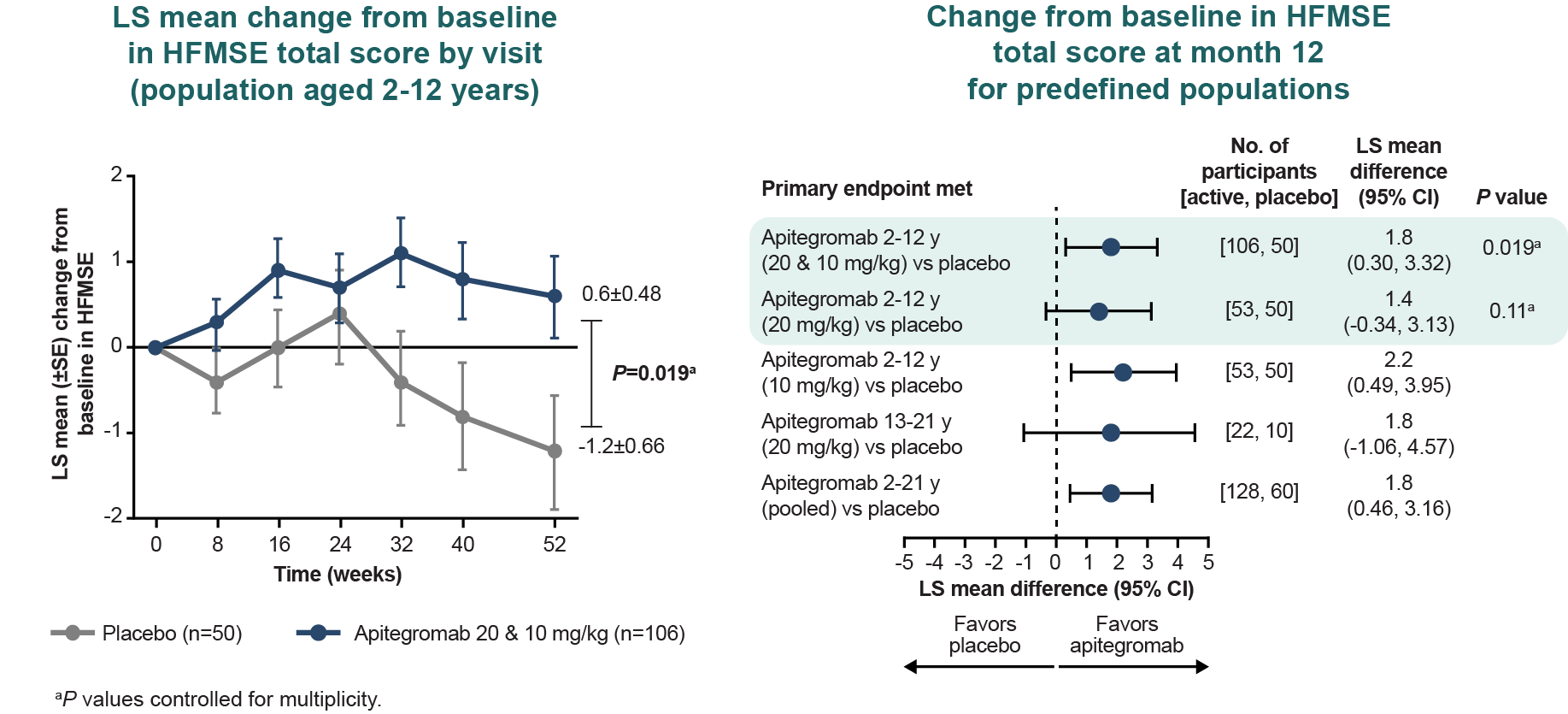
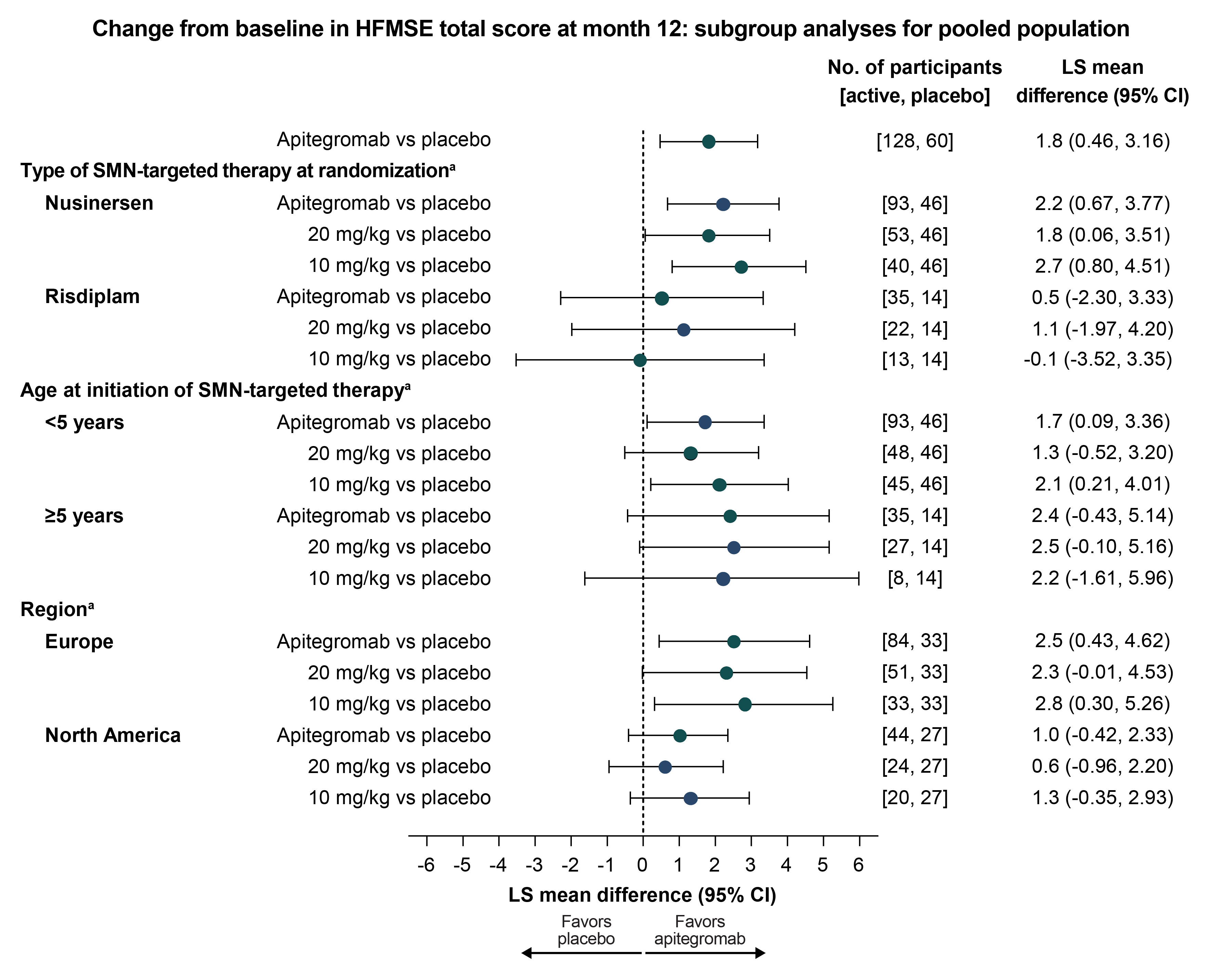
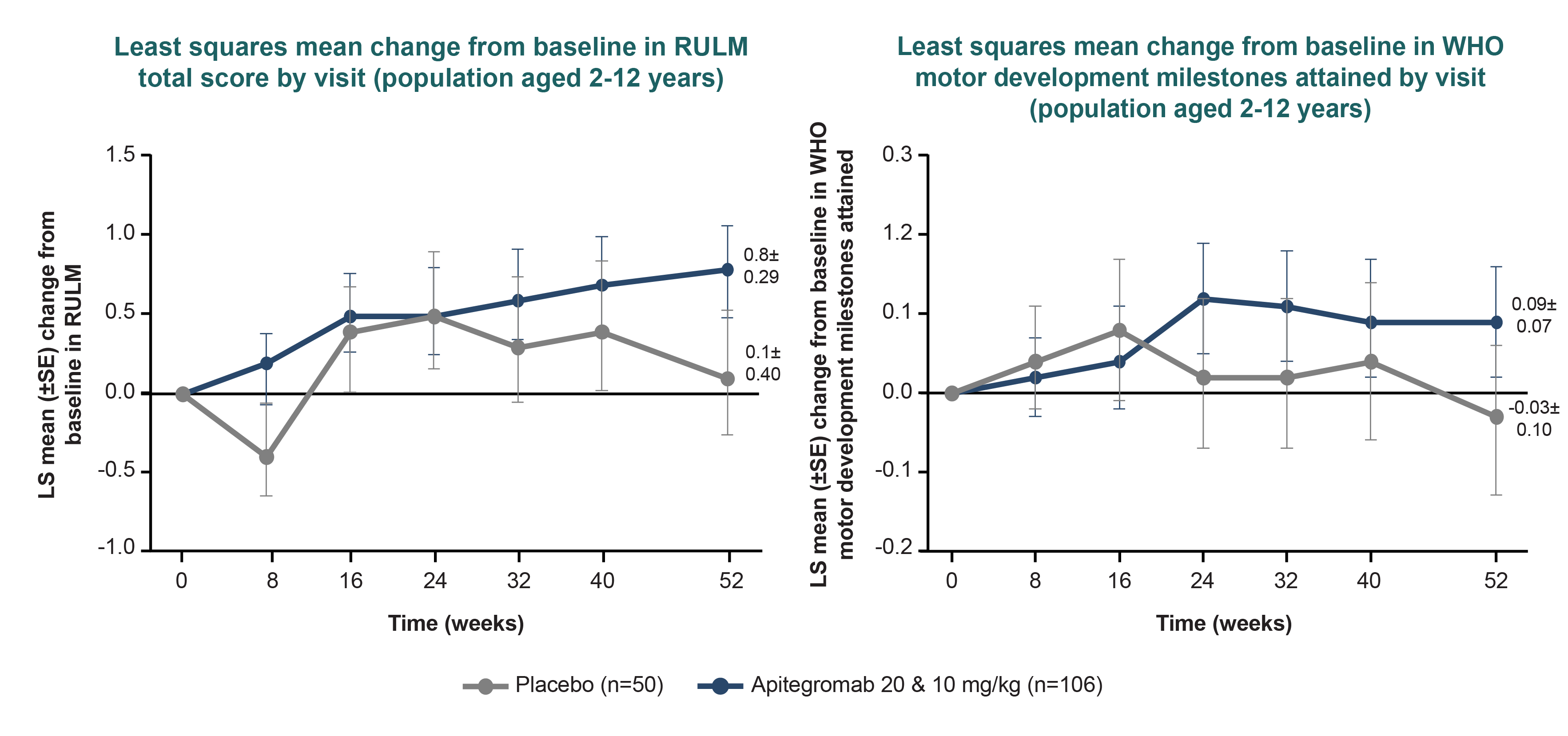
The effect size observed in participants on risdiplam was smaller relative to those on nusinersen in the population aged 2-12 years, possibly due to the small number of participants or that they were more likely to have 2 prior SMN-targeted therapies.
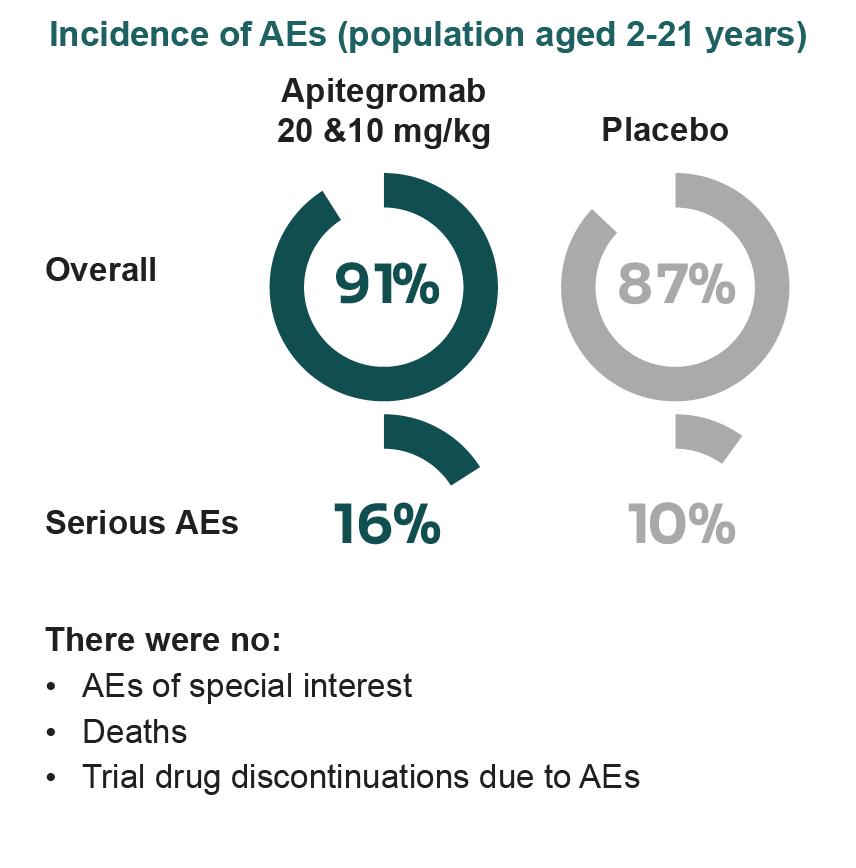
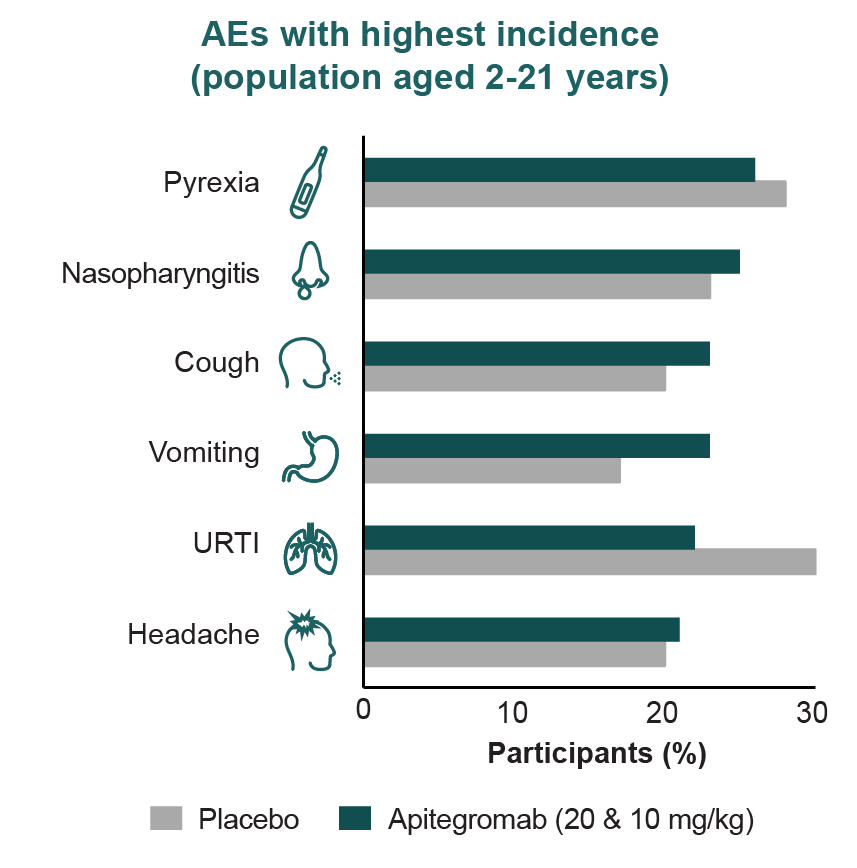
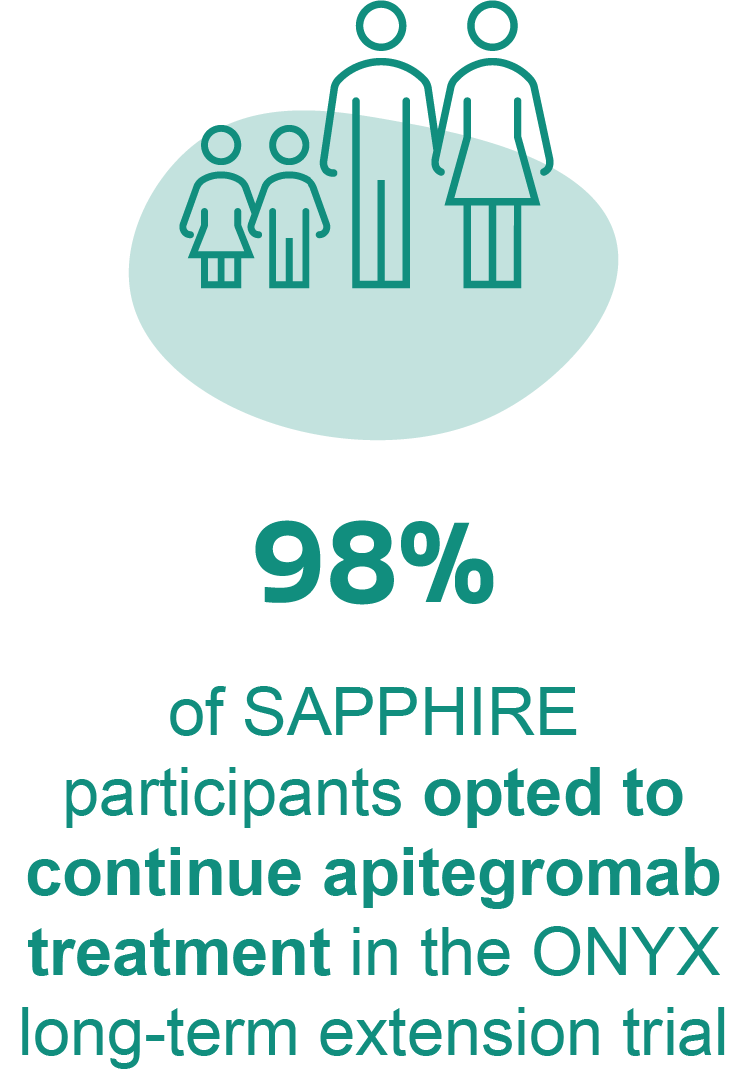
Long-term safety of apitegromab is primarily derived from the TOPAZ open-label extension, with additional data from both TOPAZ and SAPPHIRE participants enrolled in the ONYX long-term extension trial (NCT05626855).